Hi, my name is Tana-Helén. This semester I am working with the Extremophiles and Biotechnology research group in BIO299. Now I am going to tell you something incredible, and I still can’t wrap my head around it. In a terrestrial hot spring in Tajikistan a new strain of Fervidobacterium pennivorans was isolated. This bacterium has optimal growth at temperatures of 65 oC, and that makes it a thermophilic bacterium. It is also anaerobic, but the most impressive part is that this extreme bacterium can degrade feathers. But why do we spend time on investigating this bacterium and what can it do for us (López, 2018)?
Feathers, cloves, hair, nails, etc. are all outer protection structures that a range of animals use. A similarity between all of these is that they possess keratin. Keratin is common to find in nature and is a very resistant polymer, large macromolecules made up of many repetitive small molecules. In nature, keratin is slowly degraded by bacteria and fungi, but this is still a process we don’t know much about (Of and Art, 2019). But something we do know is that we humans eat a lot of chicken. According to Statista the United States produced 19.94 billion kilos of chicken and 3.4 billion kilos of turkey in 2019 (Chicken production volume U.S. 2009-2019 | Statista). But what happens with all the feathers from these productions? This huge waste often ends up in landfills, gets burned or are transformed into feather meals. Feather meals can be feed to animals, but two-thirds of the product gets discarded because of poor quality and indigestibility. Since feathers contain more than 90% keratin, we must discover a means to break it down in order to utilize this feather waste in a more sustainable manner (Of and Art, 2019). When feather meals improve in quality, they can take the place of the current ones and be added to products like fish feed. How? Perhaps a hot spring in Tajikistan has the answer.
The current situation is not sustainable—imagine tons of feathers being burned and all the gases rising into the atmosphere. And instead, we could use it feeding fish? However, this project may have a solution to all of this. The quantity of feather waste can be converted into a fine partially hydrolyzed keratin powder and fed to animals like fish rather than being burned and turned into products that are thrown away. The feathers will then be recycled and reused. Isn’t that amazing? Currently, this is not a source of keratin for people, but perhaps in the future? The project shows me that there are solutions to the climate crisis out there; we just need to figure out how to use them. It reaches multiple SDG goals (UN’s sustainable development goals) as it is an interdisciplinary, multinational project that builds on communication to achieve one common goal. This falls under SDG 17 (Partnership for the goals). The aspect of reducing waste, recycling, and all this in a natural way touches upon SDG 13 (Climate action) and 15 (Life on land). For me, this makes the project even more interesting since it may solve an important problem and be a part of the way towards a more sustainable world. (THE 17 GOALS | Sustainable Development)
So, that was the big, impressive picture of the project that I am doing a part of. The aim of my project is to conduct heterologous expression, and purify two proteases from F. pennivorans, enzymes that degrade proteins. One protease is in the family of metallopeptidases and the other is a prepilin peptidase. These proteases are expressed more strongly when F. pennivorans degrades feathers, and thus possibly play a key function but we don’t know their exact role in feather degradation. And I am going to start the work towards maybe figuring that out.
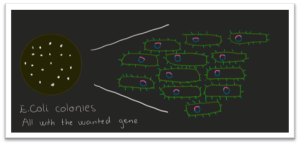
I started by amplification of these genes with PCR (Polymerase chain reaction), cloning them into a plasmid, and perform heterologous expression in Escherichia coli (Figure 5). Transformed E. coli was grown overnight, but no colonies were obtained (Figure 2). I’ve tried it again with a negative result and am trying to figure out what went wrong and how to fix it.
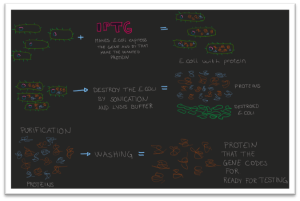
But good for me, we ordered also synthetic genes that were already inside an expression vector. I transformed this synthetic construct into E. coli and let it grow, and happily for me, I got colonies (Figure 3, 4 and 6). Now it was time for heterologous expression. I grew E. coli with my wanted gene some more and then added a solution that made the bacteria start to express the protein that my gene is coding for (Figure 7). After some time, the bacteria had made a lot of the wanted protein, so I destroyed the bacteria without harming the protein. The proteins were analyzed in an SDS-page (sodium dodecyl sulfate-polyacrylamide gel electrophoresis) gel to see if I had the right protein and, see if the protein is soluble or not. If it is, the next step is purification. With purification, the product will just be the wanted protein (Figure 7). So, I have expressed the gene and gotten the protein, and now it is possible to find out more about this protein and why and how it degrades feathers.
I appreciate having the opportunity to work for Nils Kåre Birkeland, who is in charge of this project. Additionally, I will thank Rubén Javier Lopez who has been teaching me how to operate in a laboratory and had the patience to teach me.
- Tana-Helén Meyer-Becker, autumn 2022
References:
Chicken production volume U.S. 2009-2019 | Statista (no date). Available at: https://www.statista.com/statistics/1108994/us-total-chicken-production/ (Accessed: 12 October 2022).
López, R. J. (2018) ‘Isolation and characterization of a new keratinolytic Fervidobacterium pennivorans strain from a hot spring in Tajikistan’, Master thesis University of Bergen.
Of, S. and Art, T. H. E. (2019) ‘FOSC Call 2019 addendum’, 1(3), pp. 1–20.
THE 17 GOALS | Sustainable Development (no date). Available at: https://sdgs.un.org/goals (Accessed: 15 October 2022).